We Contain Multitudes: Rethinking Human Identity Through the Microbiome
How the hologenome shapes our understanding of human evolution
Have you ever grappled with the question of what makes us human? We think we exist as singular beings equipped with free will and autonomy. But what if our microscopic companions were the ones pulling the puppet strings?
We are not individuals but walking, talking ecosystems that require feedback from our microbial symbionts and the outside world in order to regulate inflammation, train immune tolerance, maintain homeostasis. Forging ties with our microbial symbionts has allowed us to achieve what we never could in isolation.
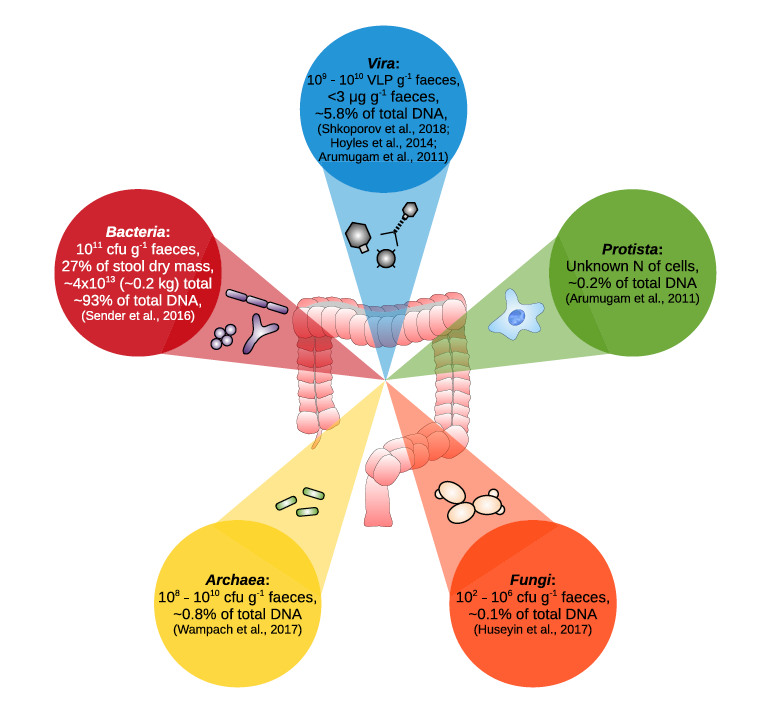
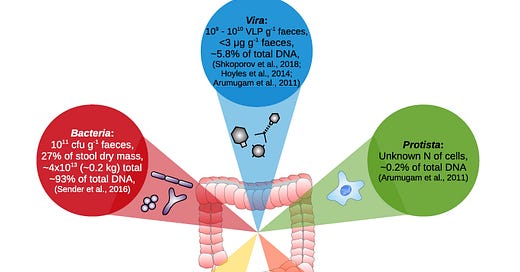
Our human chromosomes provide an incomplete picture of the genetic information we carry and neglects the bacterial, archaeal, fungal, protistan, and viral genetic information that is equally integral to human identity if not more so.
Transposable elements or transposons, often referred to as “jumping genes,” are remnants of ancestral viral and parasitic infections that subsequently became integrated into the human genome in a manner similar to the CRISPR defense system in bacteria. More than 45 percent of the human genome is derived from transposons, whereas coding regions called exons comprise less than two percent.
The Missing Link in the Evolutionary Record
Evolution has occurred at a pace much too quick to be explained by random mutation alone. To compensate, biologists have also introduced additional mechanisms such as genetic drift, gene migration, gene flow, and epistasis. However, even these mechanisms cannot sufficiently explain the quantum leaps by which evolution has taken place.
To isolate the genes of an individual as the sole drivers of evolution is to ignore the contributions of billions of years of symbiotic coexistence, at a dangerous cost. We must unite Neo-Darwinist and Lamarckian views of evolution, akin to the attempt to unite gravity and quantum mechanics in physics. To understand evolution at the macroscopic scale, we need to examine evolution at the microscopic scale as well. Enter the hologenome.
“[The hologenome concept] embraces a vibrant and more satisfying view of the nature of biology, namely that the microbiome is as essential as the genome in defining what an animal or plant is and is not.”
— Neuroscientist Roman M. Stilling
The hologenome accounts for the genetic material of a host, or holobiont, plus all its symbiont microbiota. Many bacteria that live in and on our bodies multiply rapidly enough to go through several generations of offspring within the span of 24 hours, accounting for why antibiotic resistance evolves so rapidly.
Bacteria reproduce, mutate, and therefore evolve very quickly. In this manner, the hologenome can evolve at a much more rapid pace than the human genome. As science writer Ed Yong expounds in I Contain Multitudes,
“By partnering with microbes, we can quicken the slow, deliberate adagio of our evolutionary music to the brisk, lively allegro of theirs.”
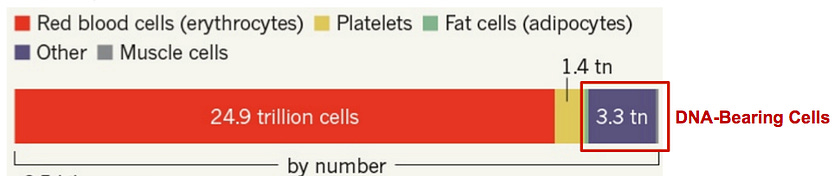
The human body has 10 times as many microbial cells as DNA-bearing human cells. A 2016 study challenged the commonly quoted 10:1 ratio of microbial cells to human cells and estimated that a human body contains ~30 trillion human cells and ~40 trillion microbes, proposing a microbe to human cell ratio of about 1.3.
However, if we are solely interested in cells bearing genetic contents and therefore ignore the non-nucleated red blood cells and platelets, we again obtain a microbial/human result of about 10:1.
Keep in mind that this ratio does not account for mitochondrial DNA, which is also hypothesized to be microbial in origin. Mitochondria are organelles responsible for cellular energy production in animals, and chloroplasts are responsible for photosynthesis in plants. Microbiologist Eugene Rosenberg, who first posited the concept of the hologenome, explains,
“Mitochondria and chloroplasts can be considered ‘extreme symbionts’ because they were derived from alphaproteobacteria and cyanobacteria, respectively.”
The human microbiome consists of 3.3 million unique genes, a set 150 times larger than our own genome. However, examining the microbiome from a purely quantitative standpoint belies the real-world utility of microbial genetic diversity.
Generally, reduction in gut microbiota diversity, such as that induced by antibiotics, leaves the host vulnerable to infection, C. difficile infection being the most well-characterized example of this phenomenon.
Loss of microbial gene diversity is observed in many diseases, and fecal microbiota transplantation, a procedure designed to restore gut microbial diversity, has been shown to be beneficial for not only gastrointestinal conditions such as C. difficile infection, inflammatory bowel disease, and irritable bowel syndrome but also autism, allergies, autoimmunity, skin conditions, chronic fatigue syndrome, depression, diabetes, obesity, and neurological disorders.
The Microbiome Compensates for Host Defects
The predictive power of the microbiome may exceed that of genome-wide association studies when it comes to understanding complex human diseases. Microbial genes provides the crucial complement to features missing in the host’s core genome.
Consider the case of lactose intolerance. Tolerance to lactose is usually assessed based on the activity of the human lactase enzyme. The lactase enzyme breaks down lactose into its monosaccharide constituents, glucose and galactose.
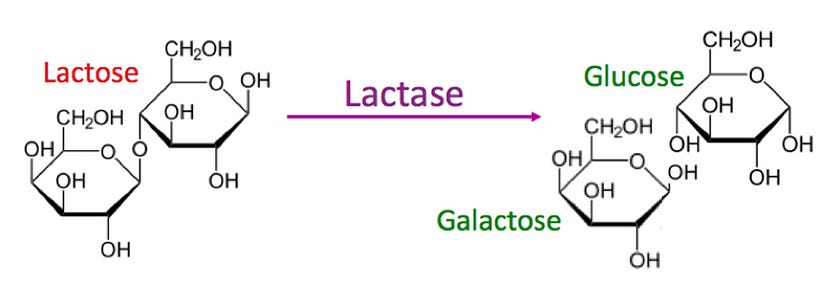
In the majority of humans, the gene that encodes for the lactase enzyme shuts off shortly after weaning, making lactase persistence rare and lactose intolerance the default status. However, many bacteria belonging to the genus Lactobacilli also have genes that encode for the lactase enzyme.
Therefore, if an individual did not have a functioning human lactase gene but nonetheless carried enough lactose-degrading symbionts, he would nonetheless be able to tolerate some amount of lactose, depending upon the activity of his fellow inhabitants’ lactase enzymes.
In addition to metabolism, microbial genes augment host fitness through major roles in detoxification, protection against pathogens, and nervous system development. In fact, the gut microbiota can exert epigenetic effects by fermenting fiber to produce metabolites such as butyrate, a short-chain fatty acid that exerts a wide range of anti-inflammatory effects.
Not only does butyrate serve as the primary energy source for cells in the colon, but butyrate is also a potent inhibitor of a class of enzymes known as histone deacetylases (HDACs), which promote the expression of genes that facilitate memory consolidation, neurogenesis, and neuroprotection.
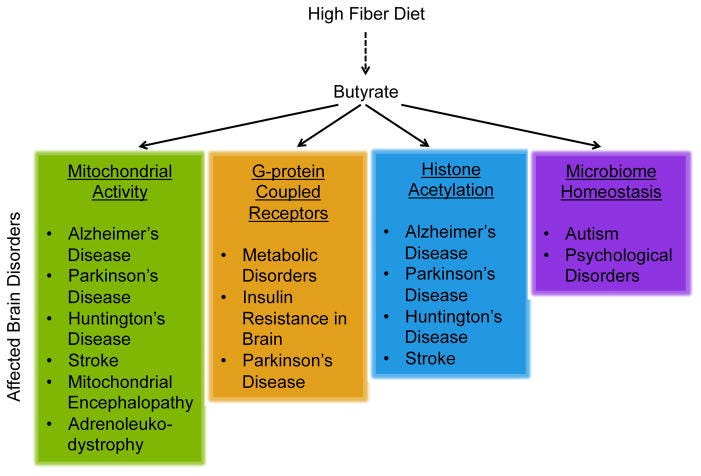
Due to its role in HDAC inhibition, butyrate is considered an anticancer agent and shows promise for the treatment of many diseases. Sodium butyrate, the salt form of butyrate commonly used in pharmacological studies, has been shown to improve brain function in animal models of Huntington’s disease, Parkinson’s disease, and advanced stage Alzheimer’s disease.
By influencing the expression of tight junction proteins, butyrate also regulates permeability of the blood-brain barrier and the gut epithelial barrier, both of which are altered in autism spectrum disorders.
Invictus, Revisited
Critics of the hologenome theory question whether the microbiome can be transmitted with sufficient fidelity across generations to be considered a unit of selection in evolution. However, another school of thought argues that transmission of specific species is irrelevant as long as the metabolic processes enabled by the microbiome are conserved.
In other words, while humans harbor taxonomically unique microbial communities, the functions those communities carry out do not exhibit much variability. Evolutionary biologist W. Ford Doolittle expressed this idea in a 2017 article entitled, “It’s the song, not the singer,” an inversion of a popular Rolling Stones song.
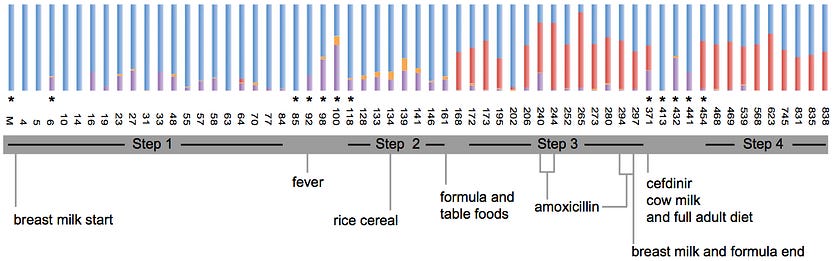
Revisiting the question of who is really in charge, researchers at Cornell University tracked the changes in the gut microbiome of a newborn for over two years and made a tantalizing discovery.
Metagenomic analyses revealed that polysaccharide-digesting functional genes appeared in the gut microbiome while the baby was still exclusively consuming breast milk, several weeks prior to the introduction of polysaccharide-containing solid food in the diet, suggesting a microbial priming of the infant gut in preparation for an adult diet.
This metabolic preprogramming implies that perhaps our microbes are the ones pulling the puppet strings, a humbling insight in the context of our anthropocentric view of the world.
Through our partnerships with our resident microbes, we have transformed so quickly as to become unrecognizable to our former selves. Though we may have spent most of our lives blind to the microscopic bonds we have formed, the interactions have nevertheless left indelible imprints on our lineage.